Infectious Disease
Lovelace Biomedical’s preclinical infectious disease research program is actively developing and refining enhanced in vitro and in vivo capabilities to improve our understanding of the host response to infection, as well as new and enhanced systems to evaluate medical countermeasures against recurring and emerging biological threats. These capabilities serve to positively impact human health through the evaluation of vaccines, therapeutics, and other medical countermeasures against bacterial, viral, fungal, and toxin exposures.
Capabilities include:
- Production of highly characterized viral, bacterial and fungal challenge stocks for use in evaluation of medical treatments and countermeasures
- Viral and bacterial neutralization and clearance assays using standard titer techniques, serological assays, as well as PCR-based and immunofluorescence methods
- High-resolution histopathology, morphometry, in situ hybridization, and immunohistochemistry with epithelial cell markers for distinct lung cell populations
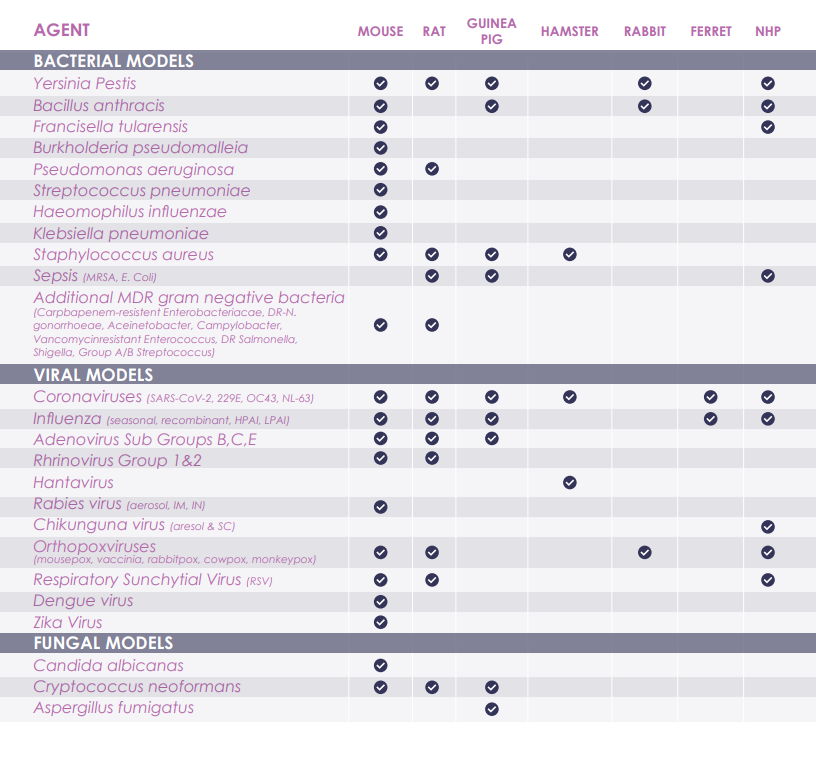
Infectious Disease Models
Lovelace Biomedical is a globally recognized leader in treatment and regulation against infectious diseases. Our team conducts a full range of infectious disease research studies under both Good Laboratory Practice (GLP) or non-GLP guidelines. We have the capability of exploring a variety of infectious diseases with our ABSL 1, 2, 3 & 3+ facilities. We integrate quality, leading-edge expertise, and an experienced and well trained staff to meet study goals.
Our infectious disease models are state of the art, allowing us to both explore the pathogenesis of disease as well as applying them to develop novel prevention and therapeutic strategies. Our professional staff has a vast experience in programs which include both In Vivo and In Vitro components. We use these methods, and other capabilities in order to fully understand the host’s response to infection, and also evaluate new medical countermeasures against recurring and emerging biological threats. We strive to serve human health through the evaluation of vaccines, therapeutics and other countermeasures targeting infectious diseases.
COVID-19 Research
Pandemics are no stranger to Lovelace Biomedical. We have previously worked on studies to find vaccines for SARS and other Coronavirus strains. Now, with COVID-19 needing a cure, we have mobilized our teams to start aiding in the fight against SARS-CoV-2 (the virus that causes COVID-19), as we did for the 2003 SARS virus.
Lovelace is leveraging its past experience with SARS-CoV1 to rapidly engage key resources (bio containment facilities, personnel, commercial and academic partners, etc) to support SARS-CoV2 research and drug development efforts.
Understanding the pathogenesis of COVID-19 in an ever changing environment and then translating that into effective models/tools that can be reliably used for testing therapeutics and vaccines does not happen overnight. This effort requires the integration of existing resources that are refocused to address the present situation and the development of new resources.
Lovelace Biomedical is a globally recognized leader in treatment and regulation against infectious diseases. Our team conducts a full range of infectious disease studies under both Good Laboratory Practice (GLP) or non-GLP guidelines. We have the capability of exploring a variety of infectious diseases with our ABSL 1, 2, 3 & 3+ facilities. We integrate quality, leading-edge expertise, and an experienced and well trained staff to meet study goals.
Lovelace has engaged multiple partners from both government and commercial sectors to develop reliable preclinical resources to expedite the development of novel therapeutics and vaccine targeting COVID-19.
See the FDA guidance here where Lovelace Biomedical’s scientists have contributed
18 For general information, see Tepper, J. S., et al., 2016, Symposium Summary: “Breathe In, Breathe Out, It’s Easy: What You Need to Know About Developing Inhaled Drugs,” Int J Toxicol, 35(4): 376–392.
Experienced in helping find cures to pandemics like the SARS virus and diseases like COVID-19
We have a program in place, and our team is ready to deploy your studies for development in animal models used to evaluate medical countermeasures for SARS-Co V-2.
View Our Published Papers
Journal of Virology
Primary Severe Acute Respiratory Syndrome Coronavirus Infection Limits Replication but Not Lung Inflammation upon Homologous Rechallenge
March, 2012
NIH.gov – PubMed
Severe acute respiratory syndrome-coronavirus infection in aged nonhuman primates is associated with modulated pulmonary and systemic immune responses
March, 2014
Our ABSL-3 Facility
Lovelace Biomedical has over 10,000 square feet of ABSL3-enhanced laboratory space, with six multi-species animal rooms capable of housing rodents, rabbits, ferrets and non-human primates. The high-containment labs are fully GLP compliant with remote telemetry capabilities, two aerosol exposure laboratories, a state-of-the art necropsy suite with full hematology and clinical chemistry analysis capabilities, as well as fully equipped microbiology and virology laboratories.
With the current state of the health crisis the world is facing in Covid-19, (SARS-CoV-2 Coronavirus), we are well equipped and able to host studies using our ABSL-3 labs to study your compounds. We specialize in these highly contagious and dangerous viral studies. Our extensive research history on deadly and dangerous diseases includes many other other Viral, bacterial, and fungal models, such as Zika, Anthrax and Crytpococcus.

